By Annabel Slater Contributors Hussein Abkallo Lucilla Steinaa February 21, 2024, In 2019, Michael Creed, Ireland’s Minister for Agriculture, had a specific fear – ‘a simple ham sandwich’.
The minister was warning that people bringing pork products back from holiday could unwittingly help spread a fatal disease of pigs. And he wasn’t alone in his warning. In the same year, the UK Department for Environment, Food and Rural Affairs (DEFRA) also launched awareness campaigns against foreign pork meat at airports, while European countries struggled to contain outbreaks of the disease—African swine fever.
African swine fever (ASF) is a viral disease which is nearly always fatal in pigs. Long endemic to Africa, in recent decades the rest of the world has woken up to its threat. It presents a huge threat to pig farming, causing death as soon as four days after infection and is easily spread through contact with animal bodily fluids, including exhaled saliva droplets.
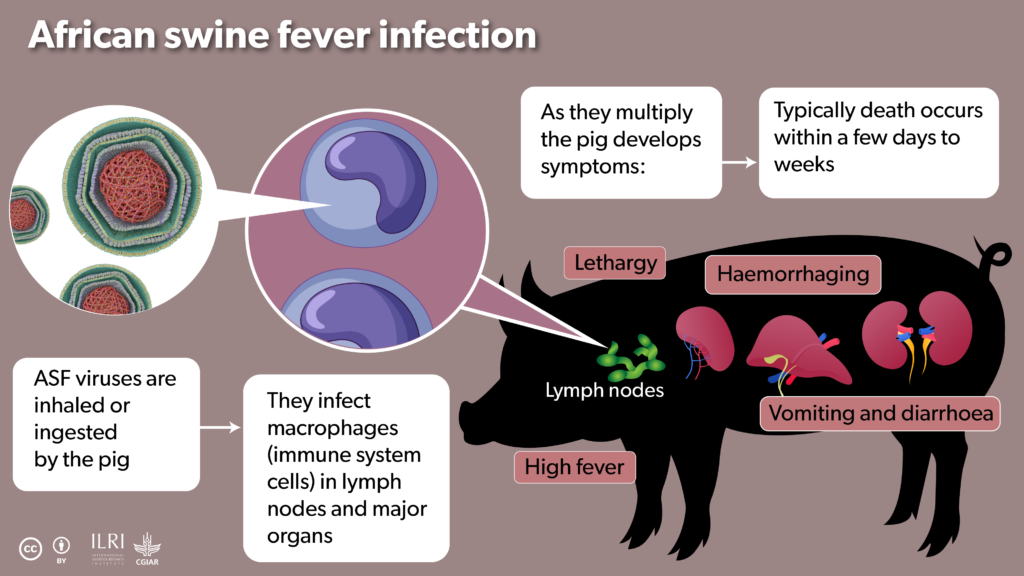 ILRI/Annabel Slater
ILRI/Annabel Slater
ASF was first scientifically described in 1921 in Kenya by veterinary pathologist Robert Eustace Montgomery, who observed that pigs brought to Kenya often died soon thereafter. But in 1957, ASF escaped the continent, and it has continued to repeat this feat until it can now be found in countries of Europe, the Caribbean and Asia.
There has never been an effective vaccine against ASF. Without one, today the disease has become a deadly pandemic. The only way to control its spread has been through strict biocontrol measures, including rapid culling of livestock. After ASF reached China in 2018, 1.1 million pigs were culled in the following year. In smallholder farms, loss of pigs can be catastrophic for household finances and nutrition.
But now, International Livestock Research Institute (ILRI) researchers believe they are on the cusp of an exciting scientific milestone.
‘We have a vaccine candidate that shows 100% effectiveness in controlled experiments with a very favourable safety profile,’ says ILRI principal scientist Lucilla Steinaa, who is leading the institute’s proprietary ASF vaccine research.
A hardy foe
First, know your enemy. ASF virus is remarkably resilient, able to survive from days to months within the soil of pigpens, pork meat and pig products, or on people’s clothing. Curing meat won’t necessarily kill the virus. Neither will repeated freezing and thawing or cooking at 56 degrees Celsius for over an hour. This is a huge problem in a world of international travel and trade, let alone other troubling risk factors such as illegal meat trading. ASF virus’s first escape in 1957 occurred because airline food waste was fed to pigs near Lisbon airport, Portugal.
 ASF virus visualisation (ILRI/Annabel Slater)
ASF virus visualisation (ILRI/Annabel Slater)
In parts of Africa and Europe, wild boar, warthog, pig and tick species have also helped to spread the virus, creating a wild reservoir of virus which is difficult to control. Ticks, for instance, can survive years between feedings, and still harbour infectious ASF virus.
Like all viruses, it also mutates. There are now 24 recognized major types or ‘genotypes’ throughout the world, and all of these genotypes have been reported in Africa. The virus is extremely good at evading the host’s immune system—and is quite difficult to make a vaccine for.
The vaccine builders
Pork is reportedly the world’s most widely eaten meat, and consumption is set to increase by 155% in sub-Saharan Africa alone by 2030. In recent years, institutes in the Global North have joined the race to develop an effective vaccine as ASF reached their shores. But in eastern and central Africa, where the most common strain of ASF is genotype IX, ILRI scientists have been working on ways to accurately diagnose ASF and understand immune responses since 2003. Then in 2017, the establishment of technologies for making a vaccine took off. And thanks to Nobel prize-winning developments in molecular biology, they’re closer than ever.
The main theory behind making an effective live-attenuated ASF vaccine is to take the virus and weaken it—make it into a form which is harmless to the pig.
 ‘When the pig gets infected, its immune system will respond to curb the virus replication. But the virus is intelligent as it demonstrates a level of adaptability. It has genes that help it evade the host immune system. By pinpointing and removing these particular genes from the virus, its ability to progress within the host and cause disease can be effectively thwarted,’ explains Hussein Abkallo, scientist at ILRI.
‘When the pig gets infected, its immune system will respond to curb the virus replication. But the virus is intelligent as it demonstrates a level of adaptability. It has genes that help it evade the host immune system. By pinpointing and removing these particular genes from the virus, its ability to progress within the host and cause disease can be effectively thwarted,’ explains Hussein Abkallo, scientist at ILRI.
Yet the attenuated virus should still be able to stimulate the pig’s immune system to provide protection against disease. Thereafter, the pig can then resist the fatal or ‘wild type’ version of the virus.
Reality is harder. An ASF virus has around 160 genes, and the function of each and how they interact with each other is extremely complex and often still unclear.
Scientists must also choose between different methods of attenuating a virus. One approach has been ‘serial passage’—infecting cells in a lab with the virus, letting it replicate, and then using those new viruses to infect more cells, and so on, and on. Under the serial influence of the cell environment, the viruses often lose genes and become less deadly. The downside is that this process is pot luck—scientists can’t control which genes will be deleted. This method has been attempted for ASF virus without any luck.
 ‘When you delete a gene, you don’t know exactly what will happen to the virus. You cannot predict it,’ says Steinaa. ‘Maybe it’s not attenuated enough and the animal still gets sick. Or it’s too attenuated so nothing happens in the pig, and then they’re not protected when they are infected with the wild type virus. You may also get completely non-productive viruses which can’t replicate if the gene that was deleted is an essential gene.’
‘When you delete a gene, you don’t know exactly what will happen to the virus. You cannot predict it,’ says Steinaa. ‘Maybe it’s not attenuated enough and the animal still gets sick. Or it’s too attenuated so nothing happens in the pig, and then they’re not protected when they are infected with the wild type virus. You may also get completely non-productive viruses which can’t replicate if the gene that was deleted is an essential gene.’
And that’s before even getting into the process of testing any of these attenuated viruses, which requires expensive, time consuming trials in live pigs. And working out the vaccine dosage, as Steinaa adds, is another challenge to unpack.
‘You have to find the right dose. If you dose too much, it might be virulent, for some deletions. Whereas there’s some deletions where you have a wide range for attenuation, so it still protects, but you can dose like 100 times more virus and the pigs are still fine. The latter is the best scenario.’
The molecular scalpel
That’s been the problem. So, what has changed?
Steinaa saw the potential of a Nobel prize-winning technology, CRISPR/Cas9 genome editing, to generate live attenuated vaccines for ASF—and other pathogens. She secured a grant from the International Development Research Centre (IDRC), co-funded by the Bill & Melinda Gates Foundation, to develop this innovative approach at ILRI.
Thanks to the grant, ILRI was able to recruit Abkallo in 2017, who had already honed his expertise in CRISPR/Cas9 through his prior research on malaria.
First published in 2012, the CRISPR/Cas9 method makes past methods of genome editing look hopelessly clumsy and slow. Using this new approach, scientists can swiftly locate any specified area in a genome and replace these sections with the precision of a molecular scalpel. Now, instead of relying on the blind process of serial passage or older, more cumbersome gene editing methods, the scientists could target known genes in the ASF genome and swiftly delete them to identify the most relevant effects.
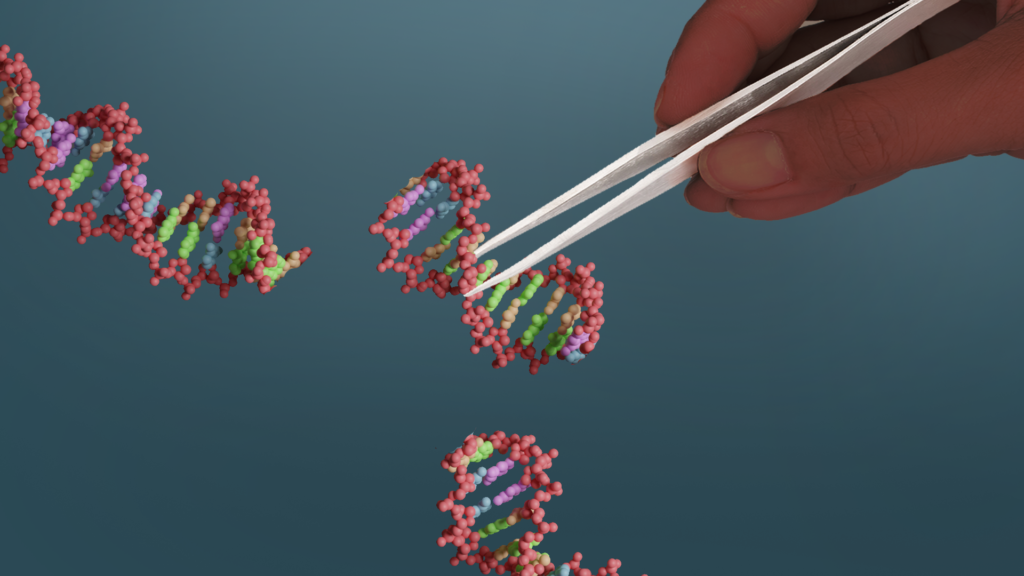 CRISPR/Cas9 is a much more precise method of genome editing than past methods. Image: ILRI/Annabel Slater
CRISPR/Cas9 is a much more precise method of genome editing than past methods. Image: ILRI/Annabel Slater
The group spent two years establishing the materials, cell lines and methods needed to use CRISPR/Cas9 editing for ASF vaccine development. And after six years their careful efforts have led to success.
‘We’ve shown that with CRISPR/Cas9, within two months you can generate multiple vaccine candidates in parallel,’ says Abkallo.
Having successfully tested the vaccine candidate in live animals for up to four weeks, ILRI scientists are now eager to find private sector partners to expand the testing towards a development phase, which will require a standardized production of the vaccine, among other things.
Abkallo says that there is a possibility a vaccine candidate could confer protection to other ASF genotypes. Or perhaps targeting the same genes in a different genotype could swiftly lead to the development of another viable vaccine. But this can only be found out through larger testing.
Steinaa and Abkallo see a lot of potential to apply CRISPR/Cas9 and ILRI’s methods to other areas of research. Genome editing can also be used to make animal breeding more accurate and efficient, making it easier to improve genetic resistance against diseases.
‘We’ve got the capacity here at ILRI for Africa, with funding, to advance this kind of research for livestock and diseases,’ Abkallo says.
‘This is really a platform technology that can be used for generating live attenuated vaccines against a variety of pathogens in the future,’ Steinaa agrees.
The vaccine candidate is a culmination of not only cutting-edge developments in molecular biology, but ILRI’s longstanding expertise in livestock research and its dedicated body of people.
‘This is really a team effort. We would have been nowhere without our excellent research technicians, research officers and the magnificent execution of the animal experiments by study managers and personnel in the clinical facility,’ concludes Steinaa.
Pork joint in Ugandan village. ILRI/Martin Heilmann
This work was funded primarily by the IDRC LVIF initiative, co-funded by Bill & Melinda Gates foundation, with co-funding from the CGIAR Program on Livestock / CGIAR Initiative on Sustainable Animal Productivity.
Source: This article was originally published on the ILRI website.







